Translation in Action: CTSA-Supported COVID-19 Work Yields High-Quality, Collaborative, Human-Centered Research Across the Translational Science Spectrum
The National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) aims to accelerate translational methods and processes that move health discoveries from bench to bedside. The COVID-19 pandemic necessitated a rapid translational process from basic research to public health impact to save lives, presenting unmatched challenges and mobilization of the Clinical and Translational Science Awards (CTSA) consortium. New evidence shows that since 2020, the CTSA consortium has effectively responded to the COVID-19 pandemic by supporting high-quality and impactful research focused on human research, working collaboratively across hubs, and responsively advancing along the translational spectrum.
In a new study published in Clinical and Translational Science, Georgia Clinical and Translational Science Alliance (Georgia CTSA) evaluators partnered with evaluation leaders from six other CTSA hubs (Boston University, Case Western Reserve University, Northwestern University, Virginia Commonwealth University, University of North Carolina, University of Wisconsin) to examine CTSA-supported COVID-19 publication metrics and trends between February 2020 and February 2023. Findings from this study validate the role of CTSA hubs as critical support structures during health emergencies and provide valuable methods for measuring impact and translation using cutting-edge bibliometric tools.
The study used innovative bibliometric methods to assess CTSA-supported COVID-19 publications for relative scientific influence, collaboration across hubs, and trends in research emphasis over time. Using NIH's iSearch COVID-19 Publication Portfolio, 3,234 peer-reviewed articles relevant to COVID-19 were identified as citing a CTSA grant, with representation from all 66 CTSA hubs. Compared to a random sample of non-CTSA-supported COVID-19 publications, the CTSA portfolio exhibited greater clinical relevance, more human research, higher Altmetric (public) attention, and higher academic citation influence. Collaboration among CTSA hubs, especially in large-scale initiatives like the National COVID-19 Cohort Collaborative (N3C), also heightened impact potential in addressing COVID-19.
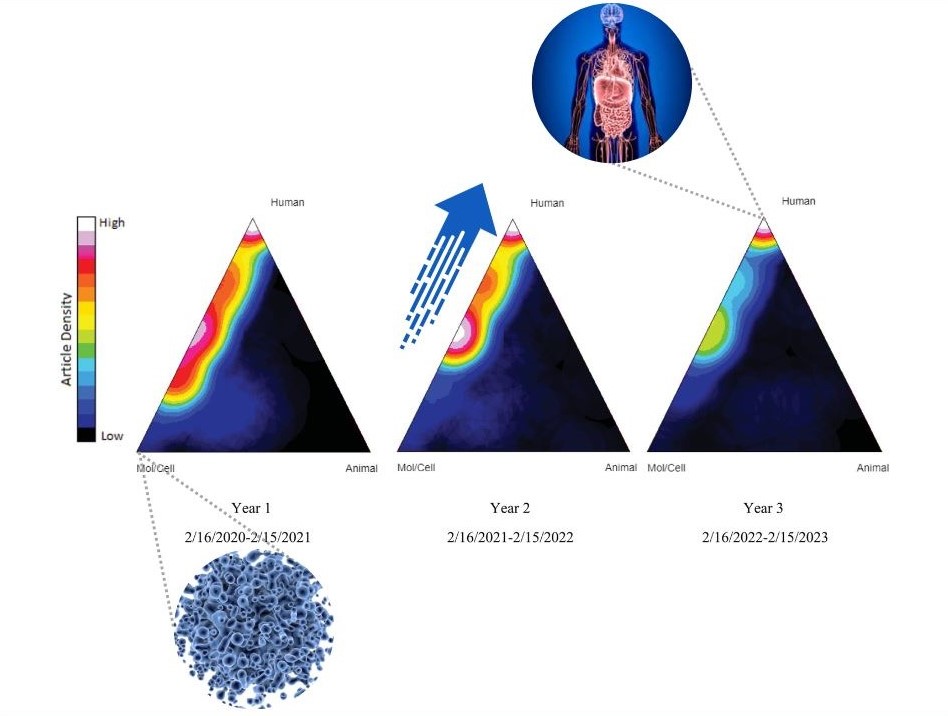
The authors utilized the Triangle of Biomedicine, part of NIH’s iCite portfolio analysis tool, to quantify and visualize the shift from basic science research toward human research in CTSA-supported COVID-19 research over time. Nicole Llewellyn, PhD, Associate Director, Georgia CTSA Evaluation and Continuous Improvement, Emory University School of Medicine, explains, “Using NIH’s iCite tool, we were able to visualize movement across the Triangle of Biomedicine away from molecular/cellular research and toward increased focus on human and public health-oriented work, demonstrating translation in action.”
“Translational research often moves too slowly and diffusely to see this kind of progression in action; but in the case of COVID-19, we were able to show that CTSA hubs helped support an influential, collaborative, and evolving body of research,” continues Llewellyn.
Aligned with the goals of NCATS, Verónica Hoyo, PhD, Executive Director, NNLM National Evaluation Center, Northwestern University, Feinberg School of Medicine shares, “This finding illustrates the mission of the CTSAs – to translate scientific discoveries into improved patient care and provide a national resource for the rapid response to urgent public health needs. Even amid the pandemic lockdowns, the CTSA consortium kept generating high-quality publications that informed clinical research.”
In addition to revealing how the CTSA consortium tackled the COVID-19 pandemic by delivering high-quality research, the study also supports NCATS’ aim toward fostering collaboration among academic institutions to improve the efficiency, quality, and impact of the process for improving human health. “CTSA-supported research has strong evidence for translation from bench to bedside more rapidly than comparative research not supported by these awards,” remarks Shannon Casey, PhD, Director, Program Evaluation and Research, Institute for Clinical and Translational Research, University of Wisconsin, Madison. “Working together, we share diverse perspectives. That collaboration helps us to innovate through shared learning and brainstorming. In collaboration, we streamline efforts rather than duplicate them. That intentionality helps increase our power as a collective.”
Eric J. Nehl, PhD, Director, Georgia CTSA Evaluation and Continuous Improvement, Emory University Rollins School of Public Health, adds, “At Georgia CTSA and across the CTSA consortium, evaluators work to show the critical impact of translational science advances. This bibliometric study was a successful collaboration between many hubs and can serve as a model for accelerating future research. It has generated a high level of interest because it demonstrates a progression from the earliest phases of COVID-19 research to a focus on prevention and impact on communities we serve.”
The publication, Translation in Action: Influence, Collaboration, and Evolution of COVID-19 Research with Clinical and Translational Science Awards Consortium Support, was published online on December 29, 2023, in Clinical and Translational Science.